News -
Novel Formulation Screening Tool
The large number of drug candidates with very poor dissolution characteristics seen in the past decade has fostered interest in amorphous solid dispersions (ASD), formulations which shall make such drugs bioavailable. Development of ASD is currently being guided by transferring the active ingredient into a solubilized state, where absorption profiles close to that of a soluble drug (BCS class I drug) can be achieved.
Formulation Challenges for Amorphous Solid Dispersions
Formulation development typically tries to achieve the most robust solubility enhancement measured by in-vitro dissolution experiments. However, a direct translation from drug dissolution to permeation is sometimes complicated by the complexity of such supersaturating systems, because the apparent concentration of a supersaturated solution is a measure of both, molecularly dissolved drug and colloidal drug, e.g. solubilized in micelles, included into cyclodextrins or polymer-bound.
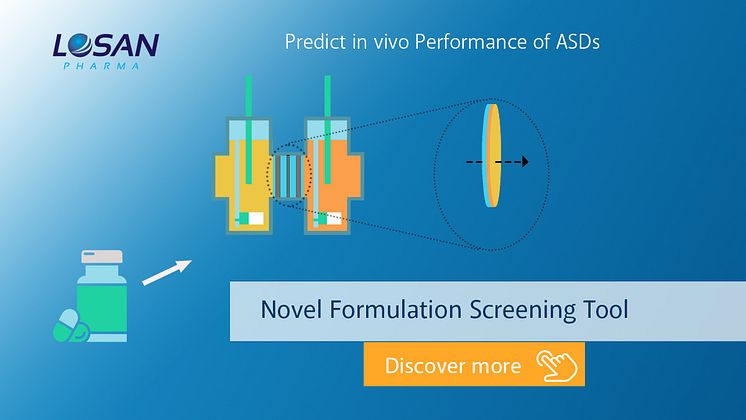
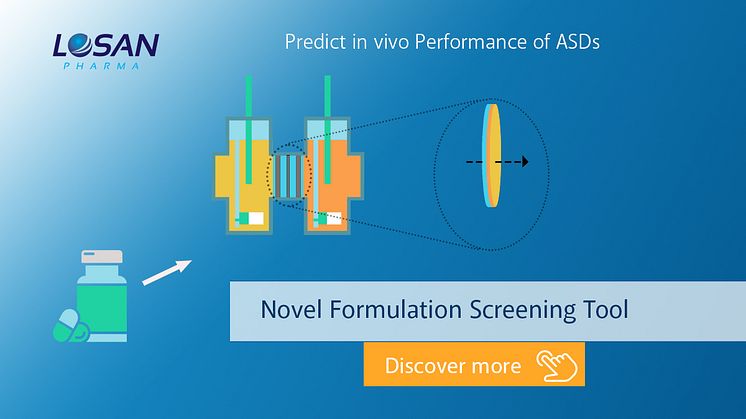
Predictive Formulations Screening Method
Losan Pharma, in cooperation with the University of Copenhagen, has established a standardized screening tool combining non-sink dissolution with permeation (D/P-setup).

The tool can aid in the evaluation of enabling formulations by strictly relying on the molecularly dissolved drug for permeation. This might reduce risk for failure in clinical trials and speed up development timelines for e.g. hot melt extrusion formulations or for ASDs gained by fluid bed layering.
Check out this publication on sciencedirect.com to view an example of a successful In vitro-in vivo correlation (IVIVC) >